PrVYZULTATM (latanoprostene bunod ophthalmic solution, 0.024% w/v) is indicated for the reduction of intraocular pressure in patients with open-angle glaucoma or ocular hypertension.1

VYZULTATM demonstrated up to 26.2% – 33.7% IOP reduction (7 – 9 mmHg) from an average baseline of 26.7 mmHg in clinical studies up to 1-year duration.1
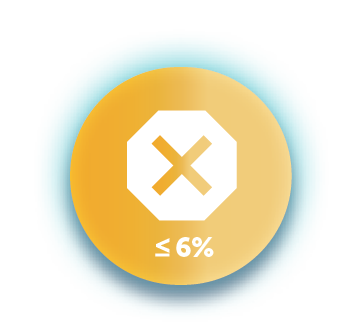
6% conjunctival hyperemia reported as the most common ocular adverse reaction. Other most common ocular adverse reactions included eye irritation (5%), eye pain (4%), and instillation site pain (2%). Approximately 0.7% discontinuation due to ocular adverse reactions.1*

32.9% of VYZULTATM patients had an IOP reduction > 25% from baseline at all 9 evaluation time points during the first 3 months of treatment vs. timolol (33% vs. 19%, p = 0.001).
Ocular adverse reactions included ocular hyperemia, conjunctival irritation, eye irritation, eye pain, conjunctival edema, conjunctivitis, vision blurred, punctate keratitis, and foreign body sensation.

Additional IOP-lowering was seen in patients who switched from timolol to VYZULTATM (1.1-1.2 mmHg, p< 0.009 for all vs. month 3).1

Demonstrated a tolerable safety profile: Ocular effects comparable to those of the first-line agent latanoprost and no significant systemic effects1